University of Kentucky UKnowledge
UKnowledge
2022
Classification and Effect of Correctors on Sitosterolemia Classification and Effect of Correctors on Sitosterolemia Associated Mutants in ABCG8
Brittney Poole
University of Kentucky, brittney.poole@uky.com
Author ORCID Identifier:
http://orcid.org/0000-0002-0955-8815 Digital Object Identifier: https://doi.org/10.13023/etd.2022.339
Recommended Citation
Poole, Brittney, "Classification and Effect of Correctors on Sitosterolemia-Associated Mutants in ABCG8" (2022). Theses and Dissertations--Medical Sciences. 23. https://uknowledge.uky.edu/medsci_etds/23
This Master's Thesis is brought to you for free and open access by the Medical Sciences at UKnowledge. It has been accepted for inclusion in Theses and Dissertations--Medical Sciences by an authorized administrator of UKnowledge. For more information, please contact UKnowledge@lsv.uky.edu.
STUDENT AGREEMENT:
I represent that my thesis or dissertation and abstract are my original work. Proper attribution has been given to all outside sources. I understand that I am solely responsible for obtaining any needed copyright permissions. I have obtained needed written permission statement(s) from the owner(s) of each third-party copyrighted matter to be included in my work, allowing electronic distribution (if such use is not permitted by the fair use doctrine) which will be submitted to UKnowledge as Additional File.
I hereby grant to The University of Kentucky and its agents the irrevocable, non-exclusive, and royalty-free license to archive and make accessible my work in whole or in part in all forms of media, now or hereafter known. I agree that the document mentioned above may be made available immediately for worldwide access unless an embargo applies.
I retain all other ownership rights to the copyright of my work. I also retain the right to use in future works (such as articles or books) all or part of my work. I understand that I am free to register the copyright to my work.
REVIEW, APPROVAL AND ACCEPTANCE
The document mentioned above has been reviewed and accepted by the student’s advisor, on behalf of the advisory committee, and by the Director of Graduate Studies (DGS), on behalf of the program; we verify that this is the final, approved version of the student’s thesis including all changes required by the advisory committee. The undersigned agree to abide by the statements above.
Brittney Poole, Student
Dr. Gregory A. Graf, Major Professor
Dr. Melinda Wilson, Director of Graduate Studies
CLASSIFCATION AND EFFECT OF CORRECTORS ON SITOSTEROLEMIA ASSOCIATED MUTANTS IN ABCG8
________________________________________
THESIS
________________________________________
A thesis submitted in partial fulfillment of the requirements for the degree of Master of Science in the College of Medicine at the University of Kentucky
By
Brittney Poole
Lexington, Kentucky
Director: Dr. Gregory A. Graf, Professor of Pharmaceutical Sciences
Lexington, Kentucky
Copyright © Brittney Poole 2022
http://orcid.org/0000-0002-0955-8815
BSTRACT OF THESIS
CLASSIFICATION AND EFFECT OF CORRECTORS ON SITOSTEROLEMIA ASSOCIATED CYTOSOLIC MUTANTS IN ABCG8
Objective: To classify mutants of ABCG8 identified in subjects with clinically confirmed Sitosterolemia, a rare form of Familial Hypercholesterolemia distinguished by the accumulation of phytosterols in plasma and tissues and determine the effects of correctors and/or regulators of proteostasis on maturation of the ABCG5/ABCG8 sterol transporter.
Methods: Disease-causing missense mutants within the cytosolic domain of ABCG8 were generated through site-directed mutagenesis. Normal and mutant proteins were expressed in human hepatocytes. Cellular proteins were prepared, and maturation was assessed by SDS-PAGE and immunoblotting. Formation of the higher molecular weight, mature form of glycoproteins was used as a bioassay for trafficking the G5G8 complex beyond the Endoplasmic Reticulum. The impact of correctors and regulators of proteostasis on Class II mutant maturation was also determined.
are maturation incompetent. Of those which matured beyond the ER, 60% were not able to traffic to the cell membrane. Of the mutants that did not mature, none were able to be rescued by small molecular chaperones (correctors).
Conclusion: HuH-7 cells are an efficiently transfected cell line that provides a system to manipulate ABCG5 and ABCG8 to make conclusions about protein maturation and trafficking to the cell surface. These experiments gave insight into the complexity of diseases caused by genetic mutations and the underlying mechanism of loss-of-function mutations. Further experimentation would be required to determine the fate of the CFTR correctors and/or regulators of proteostasis in the application in cases of Sitosterolemia.
KEYWORDS: Sitosterolemia, ABC transporters, lipids, correctors, proteostasis
Brittney Poole
(Name of Student)
07/27/22
Date
CLASSIFICATION AND EFFECT OF CORRECTORS ON SITOSTEROLEMIA ASSOCIATED CYTOSOLIC MUTANTS IN ABCG8
By Brittney Poole
Gregory A. Graf
Director of Thesis
Melinda Wilson
Director of Graduate Studies
07/27/22
Date
ACKNOWLEDGMENTS
The following thesis, while an individual work, benefited from the insights and direction of several people. First, my Thesis Chair, Dr. Gregory A. Graf, who provided a space to have a “first day in the lab.” Dr. Graf was not only supportive during the research process, but also challenged me to understand the literature and develop my own scientific thinking. Next, I wish to thank the Thesis Committee: Dr. Scott Gordon and Dr. Ryan Temel. Each individual provided insights that guided and challenged my thinking. Next, I would like to thank all the lab members of the Graf and Helsley lab for their support and expertise in the lab. I would also like to acknowledge the use of BioRender to create the figures in this thesis.
In addition to the assistance mentioned above, I received equally important assistance from family and friends. To my family, Brett, Sonja, and Courtney Poole provided on-going support while navigating living in a new state alone.
TABLE OF CONTENTS
CLASSIFCATION AND EFFECT OF CORRECTORS ON SITOSTEROLEMIA
ASSOCIATED MUTANTS IN ABCG8 ................................................................................. i
ABSTRACT OF THESIS ..................................................................................................... ii
ACKNOWLEDGMENTS .................................................................................................... iii
TABLE OF CONTENTS ..................................................................................................... iv
LIST OF TABLES ............................................................................................................... vi
LIST OF FIGURES ........................................................................................................... vii
CHAPTER 1. Introduction................................................................................................. 1
1.1 Background.............................................................................................................................. 1
1.2 Cholesterol vs. Phytosterols ..................................................................................................... 4
1.3 Sterol Absorption and Excretion ............................................................................................... 5
1.4 Sitosterolemia .......................................................................................................................... 8
1.5 ABCG5/G8 Physiology .......................................................................................................... 10
1.6 ABC Transporters .................................................................................................................. 11
1.7 Proteostasis Regulation and Roscovitine................................................................................. 15
1.8 Ivacaftor and ABCC7 Potentiation ......................................................................................... 19
1.9 Statement of Hypothesis ......................................................................................................... 22
CHAPTER 2. Materials and Methods ............................................................................. 23
2.1 Materials and Methods ........................................................................................................... 23
2.1.1 Reagents ................................................................................................................................. 23
2.1.2 Cell Culture ............................................................................................................................ 23
2.1.3 GFP Assay ............................................................................................................................. 24
2.1.4 Western Botting Analysis ...................................................................................................... 24
2.1.5 In-vitro Bioassay .................................................................................................................... 25
2.1.6 Densitometric Analysis .......................................................................................................... 25
2.1.7 Immunofluorescence Microscopy ......................................................................................... 26
2.1.8 Statistical Analysis................................................................................................................. 26
2.2 Experiment I- Generation of Sitosterolemia Associated ABCG8 Cytosolic Mutants ................ 27
2.3 Experiment II- Optimization of Transient Transfection of Human Cell Lines .......................... 30
2.4 Experiment III- Mutant Maturation Assay .............................................................................. 31
2.5Experiment IV- Native ABCG5/G8 Complex Compound Screening ....................................... 33
2.6 Experiment V- Roscovitine Toxicity ...................................................................................... 34
2.7 Experiment VI- Corrector Testing of Class II Mutants ............................................................ 34
2.8 Experiment VII- Immunofluorescence of Maturation Competent Mutants .............................. 35
CHAPTER 3. Results ....................................................................................................... 36
3.1 Experiment I- Generation of Sitosterolemia-Associated ABCG8 Cytosolic Mutants ............... 36
3.2 Experiment II- Optimization of Transient Transfection of Human Cell Lines .......................... 39
3.3 Experiment III- Mutant Maturation Assay .............................................................................. 41
3.4 Experiment IV-Corrector Testing of Class II Mutants ............................................................. 45
3.5 Experiment V- Testing Regulators of Proteostasis on Native ABCG58 ................................... 46
3.6 Experiment IV- Roscovitine Dose-Response .......................................................................... 48
3.7 Experiment VI- Immunofluorescence Trafficking Assay ........................................................ 49
CHAPTER 4. Discussion ................................................................................................. 54
4.1 Limitations............................................................................................................................. 57
4.2 Future Directions ................................................................................................................... 58
REFERENCES .................................................................................................................. 60
VITA………….. .................................................................................................................. 68
LIST OF TABLES
TABLE 1.1 ABC TRANSPORTERS AND THEIR ASSOCIATED DISEASE46. .............................................................. 3
TABLE 1.2. PROPOSED SITOSTEROLEMIA CLASSIFICATION SYSTEM52 ............................................................ 15
TABLE 1.3. ROSCOVITINE AND ANALOGS STRUCTURE. .................................................................................. 18
TABLE 1.4. IVACAFTOR AND CORRECTORS STRUCTURE ................................................................................ 21
TABLE 2.5. SITOSTEROLEMIA-ASSOCIATED MUTANTS GENERATED BY SITE-DIRECTED MUTAGENESIS IN
THE CYTOSOLIC DOMAIN OF ABCG8 ......................................................................................................... 29
TABLE 4.6. UPDATED SITOSTEROLEMIA CLASSIFICATION SYSTEM FOR MUTANTS52. ..................................... 57
LIST OF FIGURES FIGURE
1.2-1 CHOLESTEROL AND PHYTOSTEROL STRUCTURE. ........................................................................ 5
FIGURE 1.4-1 DIAGRAM DEMONSTRATING THE ABSORPTION, EXCRETION, AND SECRETION PATHWAY
OF PHYTOSTEROLS AND CURRENT THERAPEUTICS FOR SITOSTEROLEMIA. ................................................... 9
FIGURE 1.6-1 STRUCTURE OF ABC TRANSPORTER FAMILIES45. ...................................................................... 13
FIGURE 2.2-1 EXAMPLE OF SITE-DIRECTED MUTAGENSIS RESTRICTION ENZYME DIGEST AND SEQUENCE
VERIFICATION (GENERATED USING SNAPGENE SOFTWARE VERSION 4.3.11). ....................................... 29
FIGURE 2.4-1 GRAPHICAL REPRESENTATION OF IN VITRO MATURATION ASSAY. ............................................ 32
FIGURE 3.1-1 ABCG8 MUTATION DIAGRAM ................................................................................................. 37
FIGURE 3.1-2. ABCG8 SEQUENCE CONSERVATION AMONG SPECIES.............................................................. 38
FIGURE 3.1-3. ABCG8 SEQUENCE CONSERVATION AMONG ABCG FAMILY MEMBERS. ................................ 38
FIGURE 3.2-1 COMPARISON OF DIFFERENT TRANSFECTION REAGENTS IN THE HUH-7 CELL LINE. ................. 40
FIGURE 3.2-2 TRANSFECTION OPTIMIZATION IN HUH-7 CELLS. ..................................................................... 41
FIGURE 3.3-1. PROTEIN MATURATION BIOASSAY DEMONSTRATING ABCG8 CYTOSOLIC MUTANTS CO
TRANSFECTED WITH ABCG5-MYC. ...................................................................................................... 42
FIGURE 3.3-2. PROTEIN STABILITY WESTERN BLOTS OF MUTANTS IN ABCG8. ............................................. 43
FIGURE 3.3-3. RESTRICTION ENZYME DIGEST ON CLASS II MUTANTS. ........................................................... 44
FIGURE 3.3-4. GEL ELECTROPHORESIS OF CDNA FROM HUH-7 LYSATES ..................................................... 45
FIGURE 3.4-1 PROTEIN MATURATION BIOASSAY WITH TREATMENT WITH PUBLISHED CONCENTRATIONS OF
CFTR CORRECTORS68. .......................................................................................................................... 46
FIGURE 3.5-1. ROSCOVITINE SCREENING ON NATIVE ABCG5/G8 COMPLEX IN HUH-7 CELLS AT 100 UM. ... 47
FIGURE 3.5-2. ROSCOVITINE SCREENING ON NATIVE ABCG5/G8 COMPLEX IN HEPG2 CELLS AT 100 UM. ... 48
FIGURE 3.6-1 DOSE-RESPONSE DATA ON ROSCOVITINE IN HUH-7 CELLS. ..................................................... 49
FIGURE 3.7-1. IMMUNOFLUORESCENCE AND IMAGES FROM THE CYCLOHEXIMIDE TIME COURSE
EXPERIMENT. .............................................................................................................................................................. 50
FIGURE 3.7-2. TIME-COURSE WITH 50 AND 100 UG/ML CHX. ........................................................................ 51
FIGURE 3.7-3 IMMUNOFLUORESCENCE IMAGES OF ABCG8 MUTANTS........................................................... 52
CHAPTER 1. INTRODUCTION
1.1 Background
Sitosterolemia is a rare form of familial hypercholesterolemia (FH) caused by two mutations in either the ATP-Binding Cassette protein (ABC) G5 or G8 gene, which is in close proximity of 375 bp between the initiation codons and share a common promotor that function in a head-to-head orientation 1,2,3. Sitosterolemia results from a lack of function for ABCG5 or G8 in the absence of their respective binding partner, which is distinguished from other forms of FH. Sitosterolemia is an autosomal recessive inherited disease that affects about 1 in 200,000 individuals; however, it is unclear how often this disease is misdiagnosed. Clinical laboratory assays fail to distinguish cholesterol from phytosterols. Consequently, plasma from a patient with Sitosterolemia would present with what appears to be elevated total plasma cholesterol in clinical lab testing. Gas or liquid chromatography is required to distinguish phytosterols from cholesterol, a technique and instrumentation often unavailable in clinical laboratories.
Individuals diagnosed with FH and Sitosterolemia similarly present with xanthomas and premature coronary artery disease. The two diseases are distinguished by dominant vs. recessive genetics, clinical presentation, and sterol composition in the plasma. FH patients present with elevated LDL cholesterol, while Sitosterolemia patients present with increased plasma phytosterol, decreased excretion of phytosterols and cholesterol, and hemolytic and blood disorders 4,5. The underlying genetic causes of FH and Sitosterolemia differ. In cases of FH, the mutations affect either LDL-R, its ligand Apolipoprotein B (ApoB100), or the machinery required for LDL/LDL-R internalization,LDL Receptor Related Protein-Associated Protein 1 (LRPAP1) or proprotein convertase subtilisin/kexin type 9 (PCKS9) while Sitosterolemia results from genetic mutations in ABCG5 and/or ABCG8.
Many ABC transporters are associated with diseases such as; Cystic Fibrosis (ABCC7), Progressive familial intrahepatic cholestasis type 3 (ABCB4), among others (Table 1.1)6. Investigation and FDA-approved drugs (Roscovitine; CFTR modulators) partially restore function to ABCC7 and ABCB4 mutants based on their underlying molecular defect. The rationale behind testing these modulators on cytosolic mutants of ABCG8 is that if these correctors and regulators of proteostasis are effective in multiple ABC transporters, then due to the evolutionarily conserved nature of the ABC transporters (Fig. 1.2-1), these may also be effective for mutants of ABCG87.
Table 1.1 ABC Transporters and their associated disease46.
ABCTransporter | Associated Disease |
ABCA1 | Tangier’s Disease/Familial Hypoapoproteinemia |
ABCA4 | Stargardt’s Disease |
ABCB2/3 | Immune Deficiency |
ABCB4 | PFIC3 |
ABCB7 | Anemia |
ABCB11 | PFIC2 |
ABCC2 | Dubin-Johnson Syndrome |
ABCC6 | Pseudoxanthoma Elasticum |
ABCC7 | Cystic Fibrosis |
ABCD1 | X-linked Adrenoleukodystrophy (ALD) |
Table 1.1 shows different ABC transporters and their associated diseases, demonstrating a wide range of diseases caused due to mutations in ABC transporters.
We hypothesize that small molecule correctors will enhance the
maturation of native G5/G8 complex and Class II, maturation deficient mutants of ABCG8.
1.2 Cholesterol vs. Phytosterols
Sterols are an essential cellular component of eukaryotic membranes, with cholesterol in animal cells and phytosterols in plant cells. Cholesterol creates rigidity and curvature to the plasma membrane of animal cells. Cholesterol is acquired through diet or is generated by de novo synthesis. Cholesterol biosynthesis is a tightly regulated process that demonstrates negative feedback inhibition. When cholesterol is in excess, it can be toxic to the cell, while cells depleted of cholesterol cannot undergo normal physiological responses, for example, receptor signaling8. The transcription factor sterol regulatoryelement-binding protein 2 (SREBP2) regulates gene expression of the enzymes responsible for cholesterol biosynthesis and uptake to maintain homeostasis9. While endogenous cholesterol synthesis is tightly regulated, dietary cholesterol ranges due to dietary
preferences and maybe a pro-atherogenic factor10.
Phytosterols are not endogenously synthesized de novo and are strictly enter from the diet. Phytosterols are structurally similar to cholesterol, but differ from cholesterol by the side chain on the D ring of the sterol backbone (Fig. 1). For this reason, chromatography and/or mass spectrophotometry is required to distinguish phytosterols from cholesterol in the plasma. Phytosterols have an observed toxicity in the body, as a study of ABCG5/ABCG8 KOmice fed a high-phytosterol showed signs of premature death, cardiac lesions, liver damage, and hepatosplenomegaly11. With a functional ABCG5/8 transporter effectively opposing absorption and excreting of phytosterols into the feces, mice and humans are not affected by high-phytosterol containing diets11. In atypical lipid panel used to measure cholesterol, cholesterol oxidase attacks the 3ß -hydroxyl group on the A ring of cholesterol. This structural feature is shared amongsterols (animal/phytosterols) (Fig. 1) 12.GC-Mass Spectrometry would be required to separate phytosterols from cholesterol in a
plasma sample.
It has been proposed that phytosterols help reduce LDL cholesterol by competing with cholesterol for intestinal absorption resulting in a modest reduction (30-50%) of cholesterol absorption and a 10% decrease in total plasma cholesterol13. The mechanism by which phytosterols compete with cholesterol for intestinal absorption is by reducing the solubilization into the mixed micelle. However, other studies suggest phytosterols promote
cholesterol secretion13,14,15 .
Figure 1.2- 1 Cholesterol and Phytosterol structure.
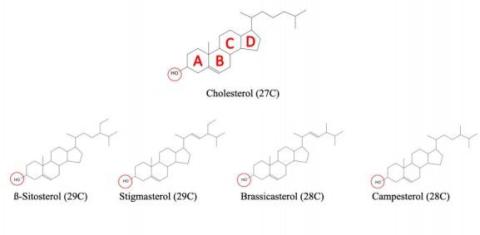
Figure 1.2- 1 Chemical structure of cholesterol and 4 of the most common phytosterols showing the differences in side chains as well as the common 3ß-Hydroxyl group. The red circle indicates common 3ß -Hydroxyl amongst sterols and ring structures are labelled in cholesterol.
1.3 Sterol Absorption and Excretion
As previously mentioned, cholesterol can enter the body in one of two ways.Cholesterol-esters and phytosterols that enter the body exogenously are metabolized by enzymes (pancreatic lipases) and emulsified with bile salts and phospholipids that enter he intestinal lumen via the gallbladder, ABCB11, and ABCB4, respectively, along with ietary cholesterol to form mixed micelles. Mixed micelles act as the "cholesterol cceptor" for dietary sterols16. Free cholesterol and hytosterols are absorbed from the ixed micelle into the small intestine (duodenum/jejunum) by PC1L1 17,18 . NPC1L1 Niemann-Pick C1 Like 1) functions at the apical surface of enterocytes and mediates the internalization of cholesterol and phytosterol to promote absorption19. In the enterocyte, holesterol is esterified by the enzyme ACAT-2 (Acyl-CoA: Cholesterol
1.4 Sitosterolemia
In 1974, two sisters presented with tuberous xanthomas and plasma plant sterols that accounted for roughly 30% of their total plasma sterols. In atypical human subject,plant sterols are typically only detected in race amounts due to poor absorption, only about 5%4. Both parents of the sisters that unaffected, leading to the conclusion that this rare lipid disease was inherited in an autosomal recessive manner4. It wasn’t until 2000 hat ABCG5 and ABCG8 ere discovered to be the two genes mutated in clinically confirmed patients with Sitosterolemia30. In 2003, it was confirmed that ABCG5 and BCG8 function as obligate heterodimers, with both proteins being required to reach the ell surface and transport sterols across the membrane31. In addition to the previously eported phenotypes of Sitosterolemic patients, subjects with clinically confirmed itosterolemia could also present with hematological abnormalities, including acrothrombocytopenia, hemolytic anemia, and splenomegaly5. urrently, the primary treatment(s) for Sitosterolemic patients are dietary odifications (low sterol diet), Ezetimibe, bile acid-binding resins, ileal ypass surgery, or LDL apheresis. However, these reatments decrease plasma phytosterols and improve ymptoms but fail to return plasma sterols to the range of a normal human subject32. zetimibe is an NPC1L1 inhibitor, reduces cholesterol, and phytosterol absorption by ~50%, and is currently the primary pharmacotherapy used to treat cases of itosterolemia33. While Ezetimibe has shown efficacy in reducing phytosterol ccumulation, the drug does not target ABCG5/G8. This is important because ABCG5/8 romotes sterol, primarily phytosterol, efflux at the enterocyte and hepatocyte apical urface, whereas NPC1L1 primarily functions in sterol absorption in the enterocyte. PC1L1 is highly expressed in both the liver and intestine and oppose biliary sterol ecretion in humans, while there is low expression mice liver19. Even if phytosterol ptake into absorption is opposed, there is stilla lack of hepatic secretion and an inability to eliminate phytosterols from plasma and tissues once accumulated (Fig. 1.4- 1). Figure 1.4- 1 Diagram demonstrating the absorption, excretion, and secretion pathway of phytosterols and current therapeutics for Sitosterolemia.
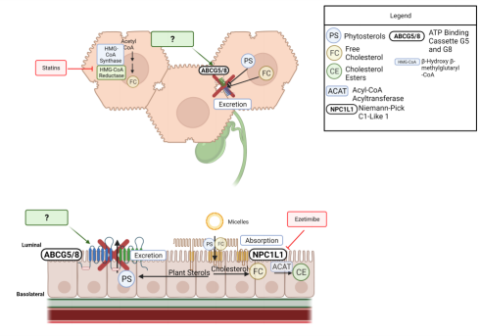
Figure 1.4- 1 Pathway for excretion and secretion of phytosterols in the liver and intestine. Diagram also exhibits the mechanism of action for ezetimibe, the most common current treatment management of Sitosterolemia.
In a 10-year follow-up study of two sisters who had a homozygous nonsense mutation (R446X inABCG5), treatment with Ezetimibe only moderately decreased phytosterol levels while diet changes had little to no change on phytosterol levels34. tarting phytosterol (sitosterol and campesterol) levels were greater than 300 µmol/L and ecreased to ~100 µmol/L, however,the normal range for a healthy individual is 10-20 mol/L34. While Ezetimibe is a form of Sitosterolemia management, these patients still ave far higher cholesterol and phytosterol absorption, which can increase the risk of ardiovascular events later in life. This demonstrates a need for a more specific harmacologic agent that targets the underlying molecular defect of ABCG5 and ABCG8 isease-causing mutants as opposed to disease management through Ezetimibe. 1.5 ABCG5/G8 Physiology
ABCG5 and ABCG8 (ABCG5/8) are two ABC-half transporters that function as a eterodimer at the apical membrane of hepatocytes (liver) and enterocytes (small intestine)30. ABCG5 and ABCG8 at the transcriptional level have been shown to be egulated by two nuclear receptors, Liver X Receptors (LXR) and Farnesoid X Receptors (FXR). LXR“ and β belong to the family of nuclear receptors that are master regulators f genes involved in cholesterol elimination pathways and form heterodimers with etinoid X Receptors (RXR)35. These transcriptional factors areactivated by cholesterol metabolites, oxysterols36. It was determined that gene expression of ABCG5 and ABCG8 was increased when mice were fed a high cholesterol diet in wildtype mice but in LXR-/- ,indicating the two genes are also regulated by the LXR/RXR transcription factor37. ABCG5 and ABCG8 can also be upregulated by the FXR pathway, a nuclear receptor ctivated in the presence of bile acids37. ABCG5 and ABCG8 are also positively regulated by the transcription factors hepatocyte nuclear factor 4(HNF4“), Forkhead box protein 1 (FOXO1), and Liver receptor homolog 1 (LRH1), but the relationships to sterol omeostasis are not as clear38,39 .